
Chancellor’s Innovation Fund Awards Support to ECE Projects
This year’s Chancellor’s Innovation Fund supports two ECE projects that include researching a better way to make EKGs wireless and faster COVID-19 antibody tests.
September 9, 2022 ![]() Isabella Mormando
Isabella Mormando
Innovation isn’t easy. Turning a research discovery into tangible technology takes time, resources and dedication.
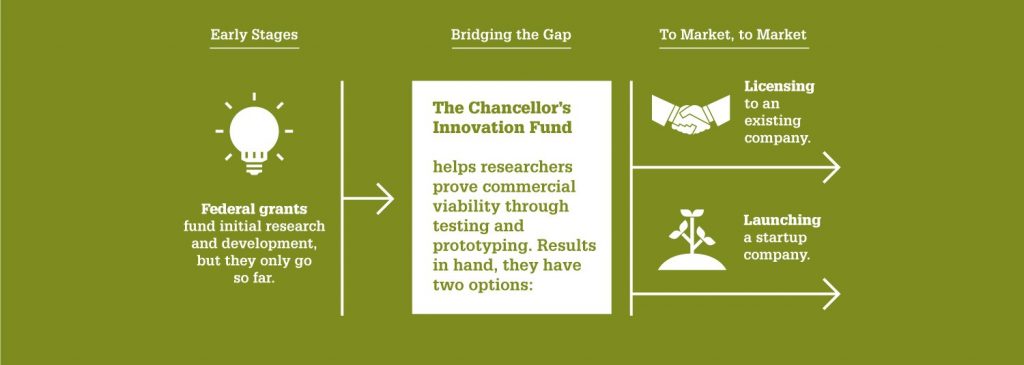
That’s why at NC State, we aim to ensure that some of our best ideas have as much support as possible. And thanks to the Chancellor’s Innovation Fund (CIF), six more projects this year will receive seed funding and support from the Office of Research Commercialization. Two of these projects come from Electrical and Computer Engineering researchers.
“The Chancellor’s Innovation Fund continues to serve as one of the most effective ways we can help our world-class faculty commercialize their cutting-edge research,” says Wade Fulghum, assistant vice chancellor of the Office of Research Commercialization. “The goal is to provide the critical funding needed to translate technologies to a point where a startup can be formed for commercialization or a license can be executed with an existing company.”
The CIF, established in 2010, awards up to $50,000 to support short-term, commercially focused research projects. Each year, a select few promising proposals are chosen based on their likelihood of market success — as well as their potential societal benefits.
The CIF seeks to help this research bridge the gap between public and private funding. For every dollar the CIF awards, it generates close to $20 in additional funding or investment.
To date, the CIF has granted nearly $3.9 million to 63 projects — which have attracted nearly $75 million in follow-on funding.
This year’s ECE projects include researching a better way to make EKGs wireless and faster COVID-19 antibody tests with the potential to revolutionize human healthcare.
Read on to learn more about the Electrical and Computer Engineering CIF recipients.
Better Bluetooth EKGs
If you’ve ever been hooked up to an electrocardiogram (EKG), you probably know how quickly its mess of long leads can get tangled amongst themselves and other sensor wires. Not only does this limit mobility, but when the wires do get crossed, any one of the average EKG’s 12 leads can easily fall off or report faulty readings.
Jordan Besnoff, a research assistant professor in the Department of Electrical and Computer Engineering, and David Ricketts, a professor in the same department, have discovered how to use “backscatter communication” to wirelessly transmit EKG information over Bluetooth to a smartphone, tablet or computer. Bluetooth EKG systems already exist, but those currently available on the market are quite bulky due to the heavy-duty batteries needed to power them. By using backscatter communication, Besnoff and Ricketts’ design eliminates the need for high-frequency signals — and in turn, any batteries at all. Transmitting the information via lower frequencies takes less energy, enabling the sensor to power itself with nothing more than on-body energy sources, such as sweat.
CIF support will be used to test an early-stage prototype of the device, including demonstrating to potential industry partners that it’s both clinically viable and medical-grade quality.
Cutting-edge COVID-19 Antibody Tests
COVID-19 antibody tests can cost hundreds of dollars and take hours to process. Today’s commercial assays require skilled personnel to run — costing as much as $1,000 per kit and taking between six and nine hours to yield results.
Stefano Menegatti, an assistant professor in the Department of Chemical and Biomolecular Engineering, and Michael Daniele, an associate professor with dual appointments in the Department of Electrical and Computer Engineering and NC State and UNC-Chapel Hill’s Joint Department of Biomedical Engineering, have developed a “dual affinity ratiometric quenching” (DARQ) assay, which aims to reduce testing turnaround time to as little as two minutes, as well as significantly lower costs — to less than $2 per test. A “simple mix-and-read” assay, Menegatti and Daniele’s technology would be ideal for both rapid testing at doctor’s offices and analytical staff in clinical or biopharmaceutical labs. The assay is also flexible. Future versions are being developed to address other antigens, which could expand the DARQ diagnostics’ reach to cancer, autoimmune diseases and degenerative disorders.
CIF support will be used to further product development with their co-inventors, graduate students Katie Kilgour and Brendan Turner. The team will produce DARQ kits for beta testing, which will include validating that the DARQ assay quantifies anti-Sars-CoV-2 antibodies in patient samples, scaling up the assay’s manufacturing, and evaluating the DARQ’s consistency and shelf-life.


